For the Love of Squalene Part II
Tom Gawriluk, PhD (tom@tritera.co)
Welcome back and thank you for checking out the second post about one of our favorite ingredients, squalene. In the last post, we discussed that squalene is a natural and unique component of human skin and as we age the amount of squalene decreases. One of Tritera’s goals is for you to replenish and maintain the natural components in your skin. Which is one reason we put squalene into all our products. In addition, there are several more wonderful biological purposes why we include squalene. I am excited to explore how squalene acts as an antioxidant and keeps our skin healthy. Please post your questions and comments below.
You may already be aware that antioxidants, like Vitamin C and Vitamin E, help keep our skin healthy. Antioxidants are naturally produced by our cells—including enzymes and metabolites—and as Davis discussed recently, we also get antioxidants from our diets as essential vitamins. Antioxidants are molecules that reduce the concentration of free radicals present in their microenvironment. For example, the skin on our face. Too many free radicals can cause inflammation and cell damage, which are bad for skin. Now did you also know that squalene is also an antioxidant, and one of the most abundant in human skin?
Last time I introduced the chemical structure of squalene and described that squalene has six carbons with double bonds. Each double-bonded carbon pair is a component of an isoprene subunit and scientific studies have shown that isoprene is an important antioxidant in plants (Loreto et al., 2001; Brunetti et al., 2015). Specifically, the double-bond acts as an electron donor to neutralize free radicals. One molecule of squalene is composed of six isoprene subunits giving it the potential to be as protective as six isoprene molecules. And because squalene is about 12% of our skin’s sebum, it follows that squalene should help reduce the concentration of free radicals in our skin.
To better understand free radicals and the damage they can do, let us take short detour. Consider that everything we can touch, smell, and see is made up of molecules. Molecules are made of atoms, which for simplicity sake are a nucleus and electrons. As you might recall from a chemistry class, negatively charged electrons orbit the nucleus and balance the electromagnetic charge of the positively charged nucleus. Of interest for this discussion, electrons are also responsible for creating covalent chemical bonds where two atoms share electrons. When an atom—by itself or as part of a molecule—is missing an electron that it would normally have, it is called a free radical. Thus, a free radical has an unbalanced positive charge, and this allows it to undergo unique chemical reactions to restore its electromagnetic balance.
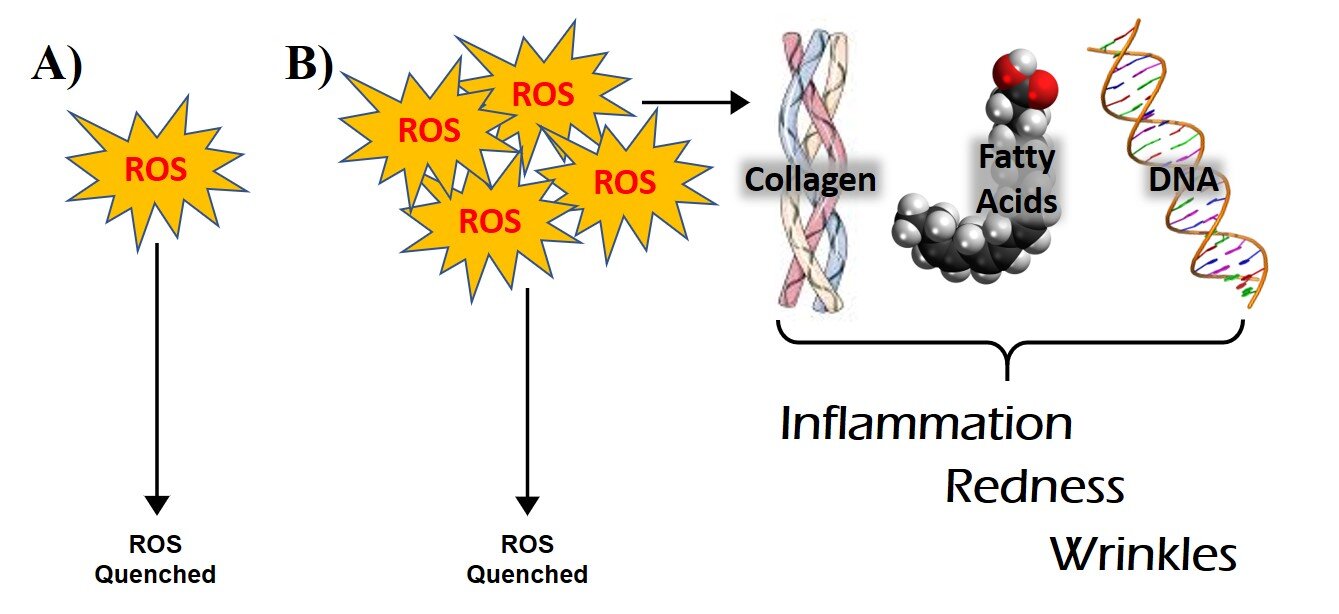
Reactive oxygen species—abbreviated as ROS—are a flavor of free radical where an oxygen atom is the free radical. ROS are naturally created in and on our skin multiple different ways and one of the most important uses is as a defense against bacteria. The concentration of ROS is controlled by our own antioxidants, for the most part. However, ROS can become abundant and problematic when skin interacts with environmental stressors including ultraviolet light and pollution. Once formed, ROS interact with another molecule by “stealing” an electron and creating a new covalent bond which makes a new molecule. When ROS interact with an antioxidant, the electron “theft” leading to a new molecule does not usually cause damage and the ROS is considered quenched. On the other hand, if the molecule that ROS interacts with is not an antioxidant, the newly formed molecule can be toxic and, in many cases, continues to create more free radicals. Importantly, when there are not enough antioxidants for ROS to interact with, the resulting reactions propagate inflammation and cause damage to important molecules, both we will now explore.
Reactive Oxygen Species and Skin: A) When ROS is produced in the skin it is normally quenched by our endogenous antioxidant system and very little damage occurs. B) If there is an accumulation of ROS from ultraviolet light exposure, pollution exposure or we do not have as many antioxidants, the excess ROS can damage collagen fibers, fatty acids and DNA leading to the appearance of aged skin.
There are three molecules in our skin that we should all worry about when it comes to ROS: fatty acids, DNA and collagen. See the cartoon below for a visual summary. When reactive oxygen species interact with fatty acids, lipid peroxides are formed. Lipid peroxide products are dangerous for two reasons. First, the end-product of lipid peroxidation is a molecule called an aldehyde, which causes inflammation. Inflammation is a complex biological condition where components of it cause tissue damage. Second, lipid peroxide products are also chemically reactive and induce a chain reaction where one lipid peroxide can react with another fatty acid producing a new lipid peroxide. This new peroxide can then also react with another fatty acid. As this chain reaction continues it harms cell membranes, kill cells and leads to tissue damage. In the skin, this contributes to sunburn, acne, and redness.
Next, when reactive oxygen species interact with DNA, the reaction can induce mutations in our genetic code. When the DNA damage occurs in a stem cell this can result in the formation of discolored spots and skin cancer. If the DNA damage occurs in our skin cells, the affected cells are usually killed by our immune system. As you might imagine, chronic ROS causes prolonged inflammation and tissue damage. And the state of inflammation can damage other structures like collagen.
Collagen is part of our skin’s extracellular matrix and individual collagen peptides assemble into helices that form a basket-like weave network throughout the dermis. This gives skin structure and strength. When the collagen network is healthy, our skin is elastic, supple and smooth. Collagen is produced by skin cell fibroblasts, which are happy when anchored to the collagen networks. When tissue is injured or damaged, for example after a cut, skin cells produce enzymes (also known as matrix metalloproteinases, or MMPs) that breakdown collagen. Among other things, this allows immune cells to get to the damaged area. Once the injury is resolved, the collagen network is fixed by destroying the MMPs and producing more collagen. This relationship works great except when the skin cell fibroblasts become unhappy. And they are unhappy when the collagen networks become fragmented causing the cells to no longer be anchored to the collagen.
The collagen damage can become extensive when ROS induced inflammation is chronic. When the collagen becomes damaged the nice tight formation begins to spread apart and this cascades into more collagen damage. As a result, our skin structure begins to break down causing wrinkle formation, sagging and rough skin, and increased inflammation over time. This all contributes to an accelerated appearance of aging skin. For more reading about ROS and skin damage, please check out this scientific review, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4496685/.
Now that you have a better understanding of how free radicals are formed and examples of the damage they cause to skin, let us get back to the role of squalene. Remember that squalene is composed of six isoprene subunits and should make squalene a good antioxidant. Here, I will now discuss some empirical data from scientific studies to support that squalene is an antioxidant.
In a study by Psomiadou, squalene was added to refined olive oil, which has no detectable squalene, and the mixture was heated. This causes environmental oxygen to react with the fatty acids in the olive oil and produce peroxide products. There was a moderate decrease in the amount of peroxide products produced in the squalene mixture when compared to refined olive oil alone (Psomiadou and Tsimidou 1999). In a different study by Kohno and colleagues, components of our sebum were separated and tested for the ability to quench a specific type of ROS called “singlet oxygen”. Squalene was the only fatty acid to show any ability to quench singlet oxygen and it also moderately prevented lipid peroxidation (Kohno et al., 1995). Furthermore, squalene was at least 1.8 times better at preventing lipid peroxidation when compared to an extract from Amaranth, a plant Chase recently discussed and is considered to have good antioxidant properties when eaten (Conforti et al., 2005). To be clear about using the adjective moderate. The antioxidant activity of squalene from these data is about 1.5% (or 66 times less than) the activity compared to the potent antioxidant Vitamin E.
Vitamin E - Vitamin C Antioxidant system: This cartoon depicts one of the most important mechanisms our skin has to reduce toxic ROS. After Vitamin E donates an electron to quench ROS, there is a chemistry feedback loop that recharges Vitamin E to continue quenching ROS. Vitamin C can donate an electron to the spent Vitamin E. Glutathione (GSH) can then recharge Vitamin C, which can then be recharged by converting NADPH to a non-toxic NADP. A serum containing Vitamin E and/or Vitamin C can help ensure that ROS do not accumulate on skin.
Vitamin E and Vitamin C are considered two of the most potent antioxidants in our skin and they are part of a synergistic electron transfer system where they can be recharged. The biochemistry is depicted in the cartoon to the left. Amazingly, when equal amounts of squalene and Vitamin E are mixed together there is also a synergistic effect! The mixture is 2.1 times more effective at preventing Croncin bleaching—a common technique to measure antioxidant properties in a test tube—when compared to Vitamin E alone (Finotti et al., 2000). This synergistic effect suggests that squalene is similar to Vitamin C and reacts with oxidized-Vitamin E recharging it to continue being a potent antioxidant.
Together these squalene studies demonstrate that squalene is an antioxidant in a test tube setting. We would also expect squalene to have antioxidant properties on our skin, too. Unfortunately, there have not been results published that directly test if the concentration of squalene in skin is associated with the amount of ROS or ROS tissue damage. However, one group tested if unrefined olive oil—which is approximately 1% squalene—can protect against skin cancer caused by ultraviolet light. Remember that when skin is exposed to ultraviolet light ROS is created, and it can damage DNA creating mutations that lead to cancer formation. The group spread olive oil onto the skin of mice and then exposed the mice to cancer-causing ultraviolet light. The results were surprising where mice treated with olive oil had less than 40% the number of tumors compared to mice treated with another oil that did not contain squalene (Budiyanto et al., 2000). Similarly, several groups have seen protective effects from testing squalene in other non-skin related cancers citing a reduction in ROS being one of the results. Additionally, one group has reported that ingesting 13 grams of squalene per day increases the amount of collagen in the skin (Cho et al., 2009). It is known that ingested squalene will accumulate in skin; however, it remains unknown how the squalene increased the concentration of collagen. I think the ingested squalene is preventing collagen destruction by acting as an antioxidant and also triggering a signaling response to increase the synthesis of new collagen. These studies help to support the hypothesis that squalene does act as an antioxidant for our skin and further supplementation is beneficial to our skin’s health.
Now that we have gone through all that information, I will try and summarize the main points.
First, free radicals like reactive oxygen species can be bad for our skin when they accumulate.
Second, the damage that free radicals do, causes our skin to become wrinkled, discolored and appear aged.
Third, antioxidants in our skin help prevent damage from reactive oxygen species—a type of free radial.
Lastly, squalene is a natural antioxidant, is synergistic with Vitamin E and likely works as an antioxidant in our skin keeping our skin healthy and young.
Tritera Optimal Antioxidant Serum reduces apparent redness and inflammation. One of our customers, who was struggling with inflamed skin, used the optimal antioxidant serum daily for two weeks. She saw a visible reduction in the redness and said her skin felt great. Additionally, her skin has a beautiful glow.
Think back to the last post. The concentration of squalene in our skin decreases as we age. It then follows that less squalene leads to more ROS damage, and that contributes to the aging of our skin. Therefore, replenishing the squalene in our skin by using a serum that contains squalene, we should be able to reduce and possibly reverse the damage caused by ROS. This gives our skin a younger appearance and makes it feel healthy.
Our group at Tritera has been creating products with this premise in mind. A product like Tritera’s Pure Squalene Serum will help replenish the lost squalene in your skin. And if you are someone that spends time in the sun or are worried about pollution, Tritera’s Optimal Antioxidant Serum was formulated to take advantage of the antioxidant synergy between squalene and Vitamin E. Now that is a pretty cool use of chemistry and biology, right? You can appreciate how well the optimal antioxidant serum works in the photos to the right.
Include Tritera products in your skincare routine and we think you will see great results!
Next time, we will go on a journey to learn more about the biological goodness of squalene. I will also go over some differences between squalene and a chemical with a similar name and chemical structure called squalane. Thank you reading and please send us your questions.
References
Budiyanto, A., Ahmed, N.U., Wu, A., Bito, T., Nikaido, O., Osawa, T., Ueda, M. and Ichihashi, M., 2000. Protective effect of topically applied olive oil against photocarcinogenesis following UVB exposure of mice. Carcinogenesis, 21(11), pp.2085-2090.
Cho, S., Choi, C.W., Lee, D.H., Won, C.H., Kim, S.M., Lee, S., Lee, M.J. and Chung, J.H., 2009. High‐dose squalene ingestion increases type I procollagen and decreases ultraviolet‐induced DNA damage in human skin in vivo but is associated with transient adverse effects. Clinical and Experimental Dermatology: Clinical dermatology, 34(4), pp.500-508.
Conforti, F., Statti, G., Loizzo, M.R., Sacchetti, G., Poli, F. and Menichini, F., 2005. In vitro antioxidant effect and inhibition of α-amylase of two varieties of Amaranthus caudatus seeds. Biological and Pharmaceutical Bulletin, 28(6), pp.1098-1102.
Finotti, E., D'Ambrosio, M., Paoletti, F., Vivanti, V. and Quaglia, G., 2000. Synergistic effects of α-tocopherol, β-sitosterol and squalene on antioxidant activity assayed by crocin bleaching method. Nahrung (Weinheim), 44(5), pp.373-374.
Jariashvili, K., Madhan, B., Brodsky, B., Kuchava, A., Namicheishvili, L. and Metreveli, N., 2012. UV damage of collagen: insights from model collagen peptides. Biopolymers, 97(3), pp.189-198.
Kohno, Y., Egawa, Y., Itoh, S., Nagaoka, S.I., Takahashi, M. and Mukai, K., 1995. Kinetic study of quenching reaction of singlet oxygen and scavenging reaction of free radical by squalene in n-butanol. Biochimica et Biophysica Acta (BBA)-Lipids and Lipid Metabolism, 1256(1), pp.52-56.
Ohsawa, K., Watanabe, T., Matsukawa, R., Yoshimura, Y. and Imaeda, K., 1984. The possible role of squalene and its peroxide of the sebum in the occurrence of sunburn and protection from the damage caused by UV irradiation. The Journal of toxicological sciences, 9(2), pp.151-159.
Psomiadou, E. and Tsimidou, M., 1999. On the role of squalene in olive oil stability. Journal of agricultural and food chemistry, 47(10), pp.4025-4032.