Vitamin E
Chase Kempinski, PhD (chase@tritera.co)
This post takes a little bit of a detour from examining a plant species and its applicability to skincare and is focused on an incredibly important antioxidant for our skin and diet—vitamin E. Antioxidants do what their name implies: they function to prevent oxidation. When we refer to antioxidants in our diet and skin care, we are generally focused on compounds that work to quench and prevent the propagation of free radicals. A free radical is a molecule containing an unpaired electron. Since electrons are stable when in pairs, a lone electron is very reactive and will pull a weakly held electron from another molecule so that it regains a pair. However, when this happens, the molecule that had its single electron removed becomes a (new) free radical. This chain reaction can cause havoc when it starts damaging proteins, DNA, cell membranes, etc. The two most common antioxidants in our cells which can quench this chain reaction are ascorbic acid (vitamin C) and tocopherol (vitamin E). It is impossible to completely prevent oxidation (and some is necessary for normal cellular function) but antioxidants serve to ensure that it does not exceed toleration.
Vitamin E chemistry and free radical formation
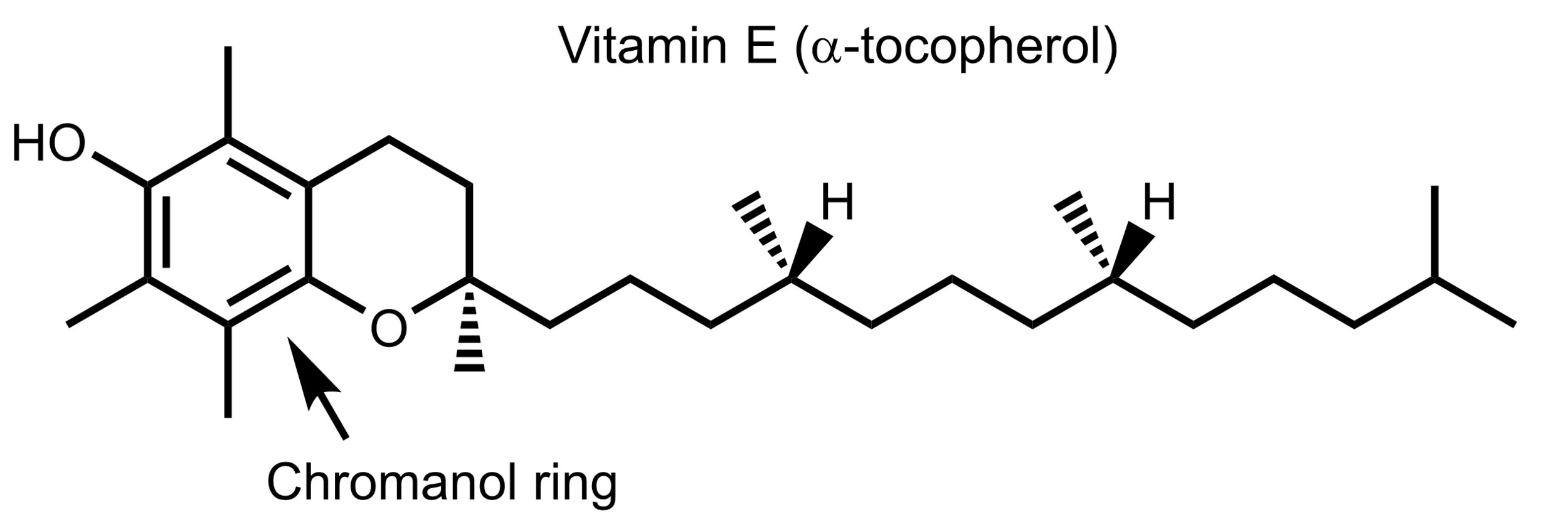
Figure 1. The structure of the most abundant isomer of vitamin E in humans, alpha-tocopherol.
Vitamin E can refer to several molecules which are all variations within the tocopherol and tocotrienol group of chemicals. Tocopherols and tocotrienols differ slightly in their structure but both are powerful antioxidants. These two chemicals are differentiated by the number of methyl groups on the chromanol ring. The chromanol ring is critical for their antioxidant activity. The forms are differentiated by the number and position of methyl groups, and are labeled α-, β-, γ-, and δ-. The most common form in humans is α-tocopherol (Figure 1). The structure of vitamin E allows it to readily donate a proton (hydrogen ion) to quench a free radical, preventing radical propagation (1). The vitamin E radical does not readily participate in radical propagation reactions because the lone electron can be stabilized over the structure of the chromanol ring. This allows vitamin E to stop radical propagation reactions and maintain the radical until it can be regenerated by vitamin C or ubiquinol.
Interestingly, one of the molecules that is present all around us contains unpaired electrons in its ground state—atmospheric oxygen. Because of this, oxygen participates in many chemical reactions (it is reactive) and seeks to gain an electron. Since oxygen was one of the first compounds observed to do this, the loss of electrons to another molecule was termed “oxidation”. Compare this to the main component of our atmosphere, diatomic nitrogen, which is essentially nonreactive. This property of oxygen is good and bad when viewed through a biological lens. It allows many of the important reactions necessary for life to take place (i.e. photosynthesis, cellular respiration) but also can cause unwanted oxidation of molecules, leading to damage and incorrect function. These compounds can be referred to as reactive oxygen species (ROS). However, there are free radicals that exist in biological systems which are generated from atoms other than oxygen, but oxygen is the most common.
Figure 2. How a hydroxyl radical can damage (peroxidate) an unsaturated lipid and cause a free radical chain reaction until quenched by vitamin E.
Most free radicals, due to their high reactivity, attack biological molecules including proteins, DNA, sugars, and lipids. Because we are specifically interested in the skin barrier (which contains oils) and the membranes of our cells (which keep their integrity), it is the protection of lipids against oxidation that specifically interests us. Vitamin E is the body’s main lipid-soluble antioxidant and an essential component of our diet. When lipids are attacked by a free radical it results in peroxidation. Vitamin E reacts with peroxyl radicals (Figure 2) a thousand-fold faster than it would react with unsaturated fatty acids. This helps to prevent the chain reaction of lipid peroxidation from continuing (2). Lipid peroxidation can cause a host of issues—at a high level, these issues can be increased inflammation (often through undesirable breakdown products), disruption of cell membranes, and raising overall oxidant stress (3). While these deleterious effects can be problems inside our bodies, they also happen on the surface.
Why do free radicals and their propagation cause so much trouble?
Our skin lipids lubricate, retain moisture, and assist in defense against the environment. There are two sources for these oils: sebaceous glands and epidermal cells. Sebaceous glands produce most of our skin oils and are comprised primarily of triglycerides, wax esters, and squalene. The epidermal cell-derived lipids act as important fillers between our skin cells with the majority of components being ceramides, free fatty acids, and cholesterol (4). Because these lipids serve as a first-line defense against the environment, they are subject to all the stresses we are exposed to—which includes UV light and pollutants. Both can trigger the generation of free radical compounds which can lead to lipid damage and the formation of breakdown products that trigger inflammation responses.
However, since vitamin E is the main lipophilic (i.e., lipid-soluble) antioxidant, it has a tremendous role in keeping the lipids on our skin in working order and preventing oxidative breakdown. A recent review paper examined studies in the literature for the propensity of vitamin E to alleviate atopic dermatitis (eczema) and found evidence that vitamin E application increased skin moisture, helped restore the epidermal barrier, reduced oxidative stress, reduced inflammation, immune dysregulation, and increased keratinocyte differentiation (outer skin cells important for protecting those below)(5). An older study, using mice, showed that topically applied vitamin E quickly penetrated down into the lower layers of skin (where the sebaceous glands reside) but still provided excellent protection against assault by ozone (6).
Vitamin E vs vitamin E acetate
You may have seen vitamin E acetate is a widely used ingredient in other cosmetics. This is a synthetically conjugated form of vitamin E to make it more shelf-stable. However, a literature review done by Thiele et al. found that vitamin E acetate is generally not as effective as vitamin E in preventing UV or oxidative-induced damage on the skin. Interestingly, vitamin E acetate is known to better penetrate the skin but must be converted to vitamin E before it can function as an antioxidant (7). The authors also state that vitamin E concentrations which are most likely to be effective in topical products are from 0.1 – 1%. Important for you (our customers!), Tritera Optimal Antioxidant Serum contains greater than 1% natural vitamin E (not the synthetic conjugate). And we believe in combination with squalene there is superior dispersion and penetration of vitamin E into your skin.
Conclusion
As mentioned, it is about the balance of antioxidant activity and the presence of reactive species that is important for healthy cells. For example, our immune system relies on the capability to produce reactive oxygen species bursts at sites of bacterial attack which help kill the invading pathogens and prevent infection (8). However, there is an abundance of evidence that shows topical application of vitamin E is safe and effective at supplementing the skin barrier. Increasing vitamin E in the skin can help protect against damage induced by UV help, pollutants, ozone, and other oxidative compounds.
References
1. E. Niki, Role of vitamin e as a lipid-soluble peroxyl radical scavenger: In vitro and in vivo evidence. Free Radic. Biol. Med. 66, 3–12 (2014).
2. M. G. Traber, J. F. Stevens, Vitamins C and E: Beneficial effects from a mechanistic perspective. Free Radic. Biol. Med. 51, 1000–1013 (2011).
3. M. Repetto, J. Semprine, A. Boveris, “Lipid Peroxidation: Chemical Mechanism, Biological Implications and Analytical Determination” in Lipid Peroxidation, (InTech, 2012), pp. 137–144.
4. A. Pappas, Epidermal surface lipids. Dermatoendocrinol. 1, 72–76 (2009).
5. C. W. L. Teo, S. H. Y. Tay, H. L. Tey, Y. W. Ung, W. N. Yap, Vitamin E in Atopic Dermatitis: From Preclinical to Clinical Studies. Dermatology 138623 (2020).
6. M. G. Traber, et al., Penetration and distribution of α-tocopherol, α- or γ-tocotrienols applied individually onto murine skin. Lipids 33, 87–91 (1998).
7. J. J. Thiele, S. N. Hsieh, S. Ekanayake-Mudiyanselage, Vitamin E: critical review of its current use in cosmetic and clinical dermatology. Dermatol. Surg. 31, 805–813 (2005).
8. L. A. Pham-Huy, H. He, C. Pham-Huy, Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 4, 89–96 (2008).